Glaucoma, a major neurodegenerative cause of blindness, is characterized by axonal injury and soma loss of retinal ganglion cells (RGCs), leading to irreversible visual impairment. Traditional optic neuroprotection strategies primarily target RGCs themselves or retinal glial cells, focusing merely on somatic survival, yet fail to restore lost visual function. The team's previous research pioneered the discovery of the upstream, critical regulatory role of retinal interneurons—amacrine cells (ACs)—in optic nerve injury. Subsequent related studies suggested that ACs might influence optic nerve regeneration via neurotransmitters, but the specific neurotransmitters and molecular mechanisms remained unclear.
On August 2, 2024, a research team led by Professors Zhuo Yehong, Li Yiqing, and Wei Yantao from Zhongshan Ophthalmic Center, Sun Yat-sen University, published a seminal article online in Science Advances titled "Modulating amacrine cell-derived dopamine signaling promotes optic nerve regeneration and preserves visual function". This study reveals a new mechanism by which dopamine derived from retinal amacrine cells regulates optic nerve injury, demonstrating that targeting retinal dopamine signaling can significantly promote optic nerve regeneration and the recovery of visual function (Figure 1).

Figure 1: Related research findings published in Science Advances
Retinal dopamine is synthesized and released by dopaminergic amacrine cells (DACs) and plays important roles in light/dark adaptation, circadian rhythms, and myopia regulation. However, the changes in retinal dopamine after optic nerve injury and its role in optic nerve regeneration remained unknown. Based on this, the research team discovered that retinal dopamine levels decrease after injury, accompanied by sustained reductions in DACs neuronal activity and the activity of tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, suggesting impaired synthesis and release of retinal dopamine. The research team found that stable, sustained supplementation of dopamine using L-DOPA, as well as enhancing DACs activity using chemogenetics, both contributed to promoting optic nerve regeneration.
Subsequently, by analyzing RGCs single-cell sequencing data, the research team identified DRD1 as the key receptor mediating dopamine's effects. Further studies using RGC-targeted Drd1 knockout mice confirmed the crucial role of DRD1. The team then demonstrated that overexpressing Drd1 in RGCs, combined with L-DOPA administration and canonical optic nerve regeneration-promoting methods (such as Pten knockout and CNTF application), achieved full-length regeneration of the optic nerve. Finally, utilizing a successfully established mouse model of chronic ocular hypertension induced by anterior chamber injection of in situ cross-linking hydrogel, the research team validated that dopamine-related therapies could partially restore visual function in mice (Figure 2).
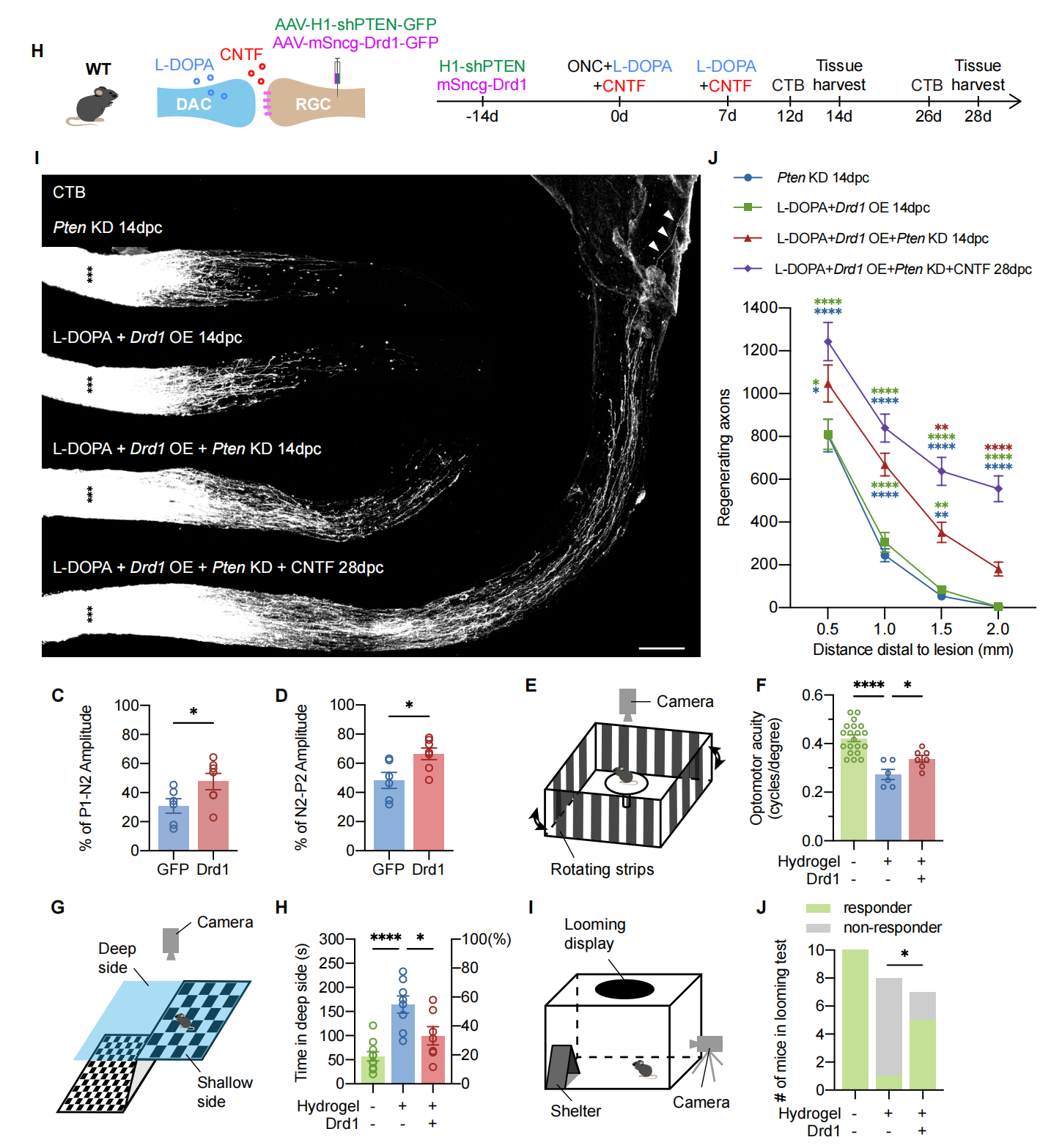
Figure 2: Dopamine-related therapeutic strategies promote optic nerve regeneration and visual function recovery in mice
Conclusion and Outlook
This study is the first to reveal the changes in retinal dopamine and dopaminergic amacrine cells following optic nerve injury, elucidates the critical role of retinal dopamine signaling in regulating optic nerve regeneration, and develops therapeutic strategies targeting retinal dopamine signaling that significantly promote optic nerve regeneration and visual function recovery. In summary, this research further refines the theoretical framework of amacrine cells in regulating optic nerve injury and repair, while also providing novel insights and a theoretical basis for the study of glaucomatous optic nerve injury and treatment.
Research Team
This study was completed by the research team of Professors Zhuo Yehong, Li Yiqing, and Wei Yantao at Zhongshan Ophthalmic Center, Sun Yat-sen University. Zhongshan Ophthalmic Center of Sun Yat-sen University / the State Key Laboratory of Ophthalmology is the sole affiliation of the authors. Professors Zhuo Yehong, Li Yiqing, and Wei Yantao are co-corresponding authors. Doctoral student Zhang Qi is the first author. This research was supported by the National Key R&D Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Guangdong Basic and Applied Basic Research Foundation.
Link to the original article: