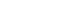With rapid advancements in new ophthalmic drugs and medical devices, every ocular examination image may hold key clues for optimizing disease treatment plans. The Imaging Reading Center at the Clinical Research Center of Zhongshan Ophthalmic Center, in collaboration with the Image Reading Center of Doheny Eye Institute (USA), adopts international standard management models and reading protocols to provide high-precision image interpretation services for ophthalmic clinical research. To date, the center has undertaken 13 ophthalmic clinical trials, completed standardized evaluations for over 80,000 eye images, and supported 5 clinical trial projects in passing reviews by the National Medical Products Administration (NMPA).
1. Three Core Strengths, Setting Benchmarks
(1) Multi-Modal Image Evaluation
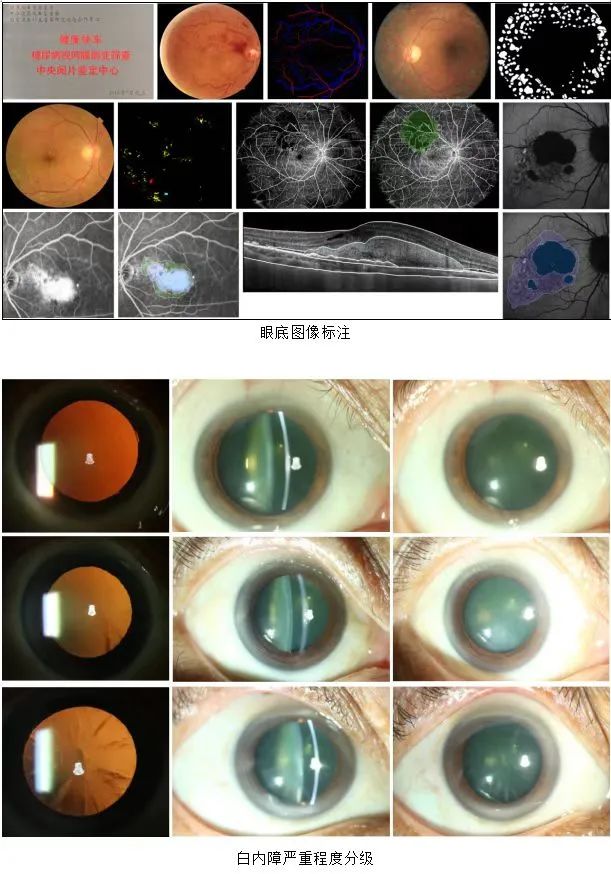
・Supports analysis of fundus photography (FP), fundus fluorescein angiography (FFA), optical coherence tomography (OCT), fundus autofluorescence (FAF), indocyanine green angiography (ICGA), and anterior segment slit-lamp photography.
・Implements an international standard double-blind cross-review mechanism to ensure objective and reliable data.
(2) Professional Ophthalmic Imaging Team
・Includes 2 senior reading consultants and 7 image reading specialists.
・Certified by the Doheny Image Reading Center (USA) and the UK Diabetic Retinopathy Screening Programme.
・Well-versed in image evaluation requirements for multicenter clinical trials.
(3) End-to-End Solutions
・Protocol Design: Customized image evaluation standards and optimized image acquisition protocols.
・Project Initiation: Standardized training for multicenter image acquisition, establishment of image transmission and quality control platforms, and creation of project-specific image databases.
・Project Execution: Real-time monitoring of image quality, technical guidance, and regular progress reports on image evaluation.
・Data Delivery: Data conversion in CDISC-compliant formats and support for regulatory data reviews.
2. Classic Success Stories, Demonstrating Excellence
Phase III Anti-VEGF Trial: Supported the approval and market launch of Conbercept for CRVO and Zhuochuming for wAMD through NMPA reviews.
Medical Device Registration: Facilitated the issuance of China’s first Class III medical device registration certificates for AI-based diabetic retinopathy and multi-disease AI solutions through standardized fundus image evaluations.
3. Strict Quality Control System, Ensuring Data Security
・Tiered Quality Control: Expert review and adjudication following double-blind readings.
・Full Traceability: Supports 7×24-hour data tracking.
・Data Security: HIPAA-compliant data storage.
Choose the Imaging Reading Center at the Clinical Research Center of Zhongshan Ophthalmic Center, — let professional image evaluation become a powerful accelerator for your ophthalmic clinical research!
Phone: +86 20 6660 4128
Email: zirc@gzzoc.com

Team of Imaging Professionals
Pan Jianying, Huang Jiamin, Li Huiting
Ou Dongmei, Ou Yiling, Wu Xiaoyi, Xie Huirui

Reading Consultants
Left: Prof. Luo Yan (Reading Consultant)
Right: Dr. Ye Huijing (Associate Chief Physician, Reading Consultant)
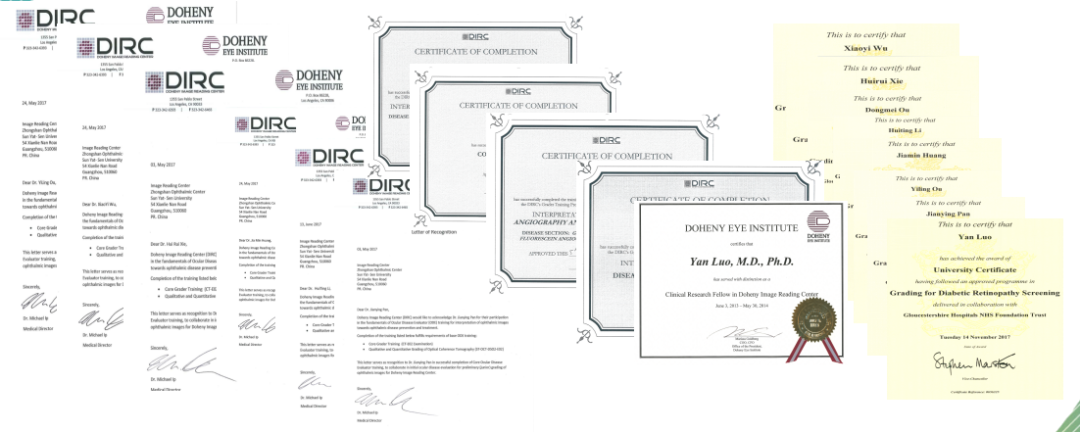
Certification