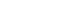Retinitis pigmentosa (RP) is a group of common, refractory blinding diseases characterized by progressive degeneration and death of photoreceptors and the retinal pigment epithelium (RPE). The causative genes and pathogenesis have not been fully elucidated. RPE cells are the crucial "support unit" for maintaining the function and survival of photoreceptor neurons. Daily photoreceptor outer segment (POS) phagocytosis and recycling their components are key functions of the RPE. Impairment of this function leads to the accumulation of debris in the subretinal space, ultimately resulting in photoreceptor degeneration and thus loss of vision. Autophagy, the internal "cleaning and recycling system" of cells, is essential for maintaining RPE health and POS phagocytosis. However, the specific regulatory mechanisms of autophagy in the RPE remain poorly understood. RP is an inherited retinal degenerative condition, whose candidate genes provide avenues for dissecting novel autophagy factors.
Recently, researchers from Zhongshan Ophthalmic Center, Sun Yat-sen University, published a research paper titled "Retinitis Pigmentosa-Associated Gene TRIM49 Regulates ULK1-Mediated Autophagy and Photoreceptor Phagocytosis by the Retinal Pigment Epithelium" in Advanced Science (CAS Q1). This study firstly report the identification of biallelic variants in TRIM49 as novel causes for autosomal recessive RP. It further elucidates a novel mechanism by which TRIM49 maintains RPE homeostasis through regulating ULK1-mediated autophagy and POS phagocytosis, offering a new potential therapeutic target for retinal degenerative diseases.
01. Identification of the Novel Gene for RP
By comparing the whole-exome sequencing data from two unrelated probands with RP and 7,283 in-house controls, combining with excluding pathogenic variants in known genes, the research team pinpointed a novel candidate gene – TRIM49. One family exhibited a unique genetic phenomenon – uniparental disomy.
TRIM49 homozygous pathogenic variants identified in two unrelated RP families
02. Impact of TRIM49 on the cellular homeostasis and POS phagocytosis capacity of human RPE
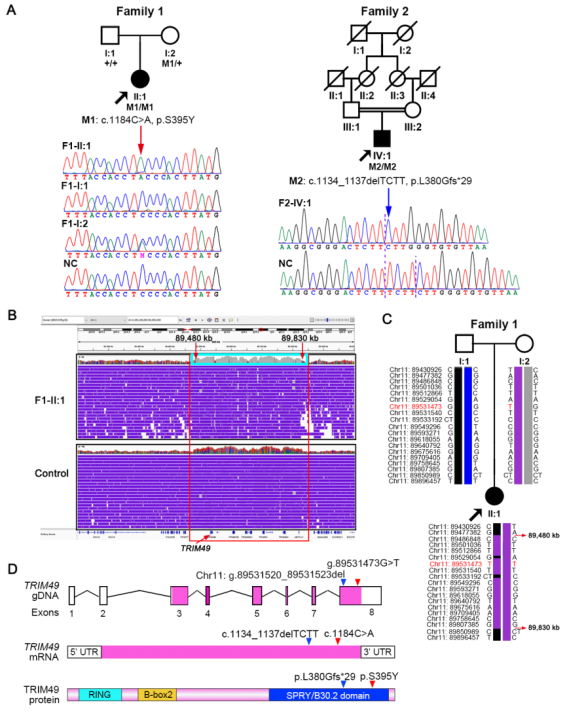
TRIM49 is a primate-specific gene involved in autophagy, with no homologous gene in rodents or other common laboratory animals. Among human tissues, TRIM49 mRNA is highest in the retina and the protein is exclusive to the RPE of the human retina.. Further functional studies of TRIM49 were conducted in human RPE cells to clarify the pathogenic mechanism. They found that TRIM49 deficiency led to a series of abnormalities in RPE cells, including elevated reactive oxygen species levels, decreased mitochondrial membrane potential, reduced ATP production, increased apoptosis rate, and Inhibited cell proliferation, indicating that TRIM49 is indispensable for maintaining the cell health of human RPE. In addition, TRIM49 deficiency significantly impairs the phagocytic activity of human RPE cells. The underlying mechanism involves TRIM49 deficiency downregulating the expression of key phagocytic receptors (such as β5 integrin and CD36) and their downstream signaling molecules, thereby hindering the "recognition" and "internalization" processes of phagocytosis.
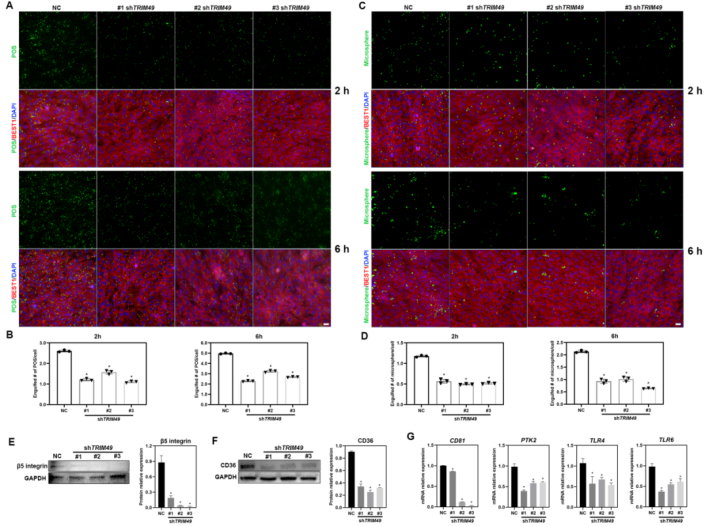
TRIM49 deficiency impairs the phagocytic capacity of human RPE and downregulates phagocytic receptors expression.
03. Mechanism Exploration: How TRIM49 Regulates RPE Phagocytic Function via Autophagy
Autophagy plays a central role in maintaining RPE POS phagocytosis, digesting and recycling POS components in lysosomes in response to light and stress conditions. It was revealed that TRIM49 is a key positive regulator of the autophagy flux in RPE, acting at the initiation stage of autophagy. In detail, TRIM49 interacts with ULK1, a core protein for autophagy initiation. TRIM49 deficiency down-regulates ULK1, thereby inhibiting autophagy initiation. Patient-derived variants impaired the positive regulatory capacity of TRIM49 on autophagy. In addition, the TRIM49 expression was revealed to be regulated by miR-548b-3p and OTX2, a key transcription factor for retinal development. They forms a complex regulatory network that collectively maintains normal RPE function.
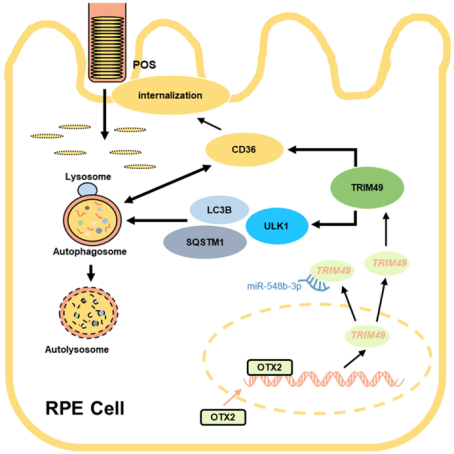
Presumed model of TRIM49 function in the RPE
In summary, based on a large Chinese cohort with various genetic eye disease and high-throughput sequencing, combined with cell models and molecular mechanism studies, this research identifies TRIM49 as a novel causative gene for autosomal recessive RP, and links autophagy, phagocytosis, and RP pathogenesis. The findings provide new perspectives for understanding the pathological processes of photoreceptor degenerative diseases (such as RP and AMD), and suggest that targeting RPE autophagy or phagocytic receptors (such as CD36) is potentially a therapeutic strategy for such diseases in the future.
Research Team Introduction
Zhongshan Ophthalmic Center of Sun Yat-sen University / the State Key Laboratory of Ophthalmology is the first and corresponding author affiliation. Ophthalmic Physician Dr. Yi Zhen, Master's student Wen Chaojuan, Master's student Du Han, and Postdoctoral Fellow Wang Yingwei are the co-first authors of the paper. Professor Zhang Qingjiong, Senior Researcher Shen Huangxuan, and Associate Researcher Sun Wenmin are the co-corresponding authors.
First Author Profile

Yi Zhen, M.D., Ph.D., is an Ophthalmic Physician in the Pediatric Ophthalmology and Ocular Genetics Department. She graduated as a direct-Ph.D. student supported by the "Excellent Student Cultivation Program" of Zhongshan Ophthalmic Center, Sun Yat-sen University, under the supervision of Professor Zhang Qingjiong, an expert in the field of pediatric and genetic eye diseases. She was recognized as an Outstanding Ph.D. Graduate of Sun Yat-sen University in 2020 and currently serves as a Youth Committee Member of the Medical Genetics Branch of the Guangdong Medical Association. She is dedicated to research on causative genes, genotype/phenotype characteristics, and molecular mechanisms of inherited eye diseases. As project leader, she has presided over five research projects, including grants from the National Natural Science Foundation of China. As first author (including co-first author), she has published eight papers in internationally authoritative journals such as Advanced Science, The Lancet Discovery Science, Zoological Research, Journal of Medical Genetics, and Investigative Ophthalmology & Visual Science.
Link to Original Article
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202512305