On the morning of October 22, 2024, Zhongshan Ophthalmic Center (ZOC) of Sun Yat-sen University held a press conference at its Zhujiang New Town Campus to announce its latest research findings. The team led by Professor Yang Huasheng from ZOC, in collaboration with Professor Qian Jiang's team from the Eye, Ear, Nose, and Throat Hospital of Fudan University, published an original clinical research paper in the Journal of the American Medical Association (JAMA). The study confirmed that "a 3-cycle CEV chemotherapy regimen achieves equivalent efficacy to the traditional 6-cycle regimen for high-risk retinoblastoma." The press conference was chaired by Professor Lin Haotian, President of ZOC and Director of the State Key Laboratory of Ophthalmology. Professor Yang Huasheng, the project leader and corresponding author of the paper, presented the research findings.

Research Overview
Retinoblastoma is the most common intraocular malignancy in children, with approximately 8,000 new cases diagnosed worldwide each year. Children with advanced retinoblastoma exhibiting high-risk pathologic features face a significant risk of intracranial and distant metastasis. These metastases often lead to rapid disease progression and pose a severe threat to life. Postoperative adjuvant chemotherapy is the primary treatment for these patients. However, due to a lack of randomized clinical trial data specifically for children with advanced retinoblastoma and high-risk pathologic features, the optimal number of chemotherapy cycles remains uncertain.
While the traditional 6-cycle CEV (carboplatin, etoposide, and vincristine) regimen is effective, its extended duration results in more adverse effects and complications, placing a heavy burden on both the children and their families. To shorten the treatment course, reduce chemotherapy side effects, and alleviate this burden, Professor Yang Huasheng's team pioneered a 3-cycle CEV chemotherapy regimen and investigated whether it demonstrated equivalent efficacy to the traditional regimen.
On October 21, 2024, the research paper titled “Three vs 6 Cycles of Chemotherapy for High-Risk Retinoblastoma: A Randomized Clinical Trial” was published in the prestigious international medical journal JAMA. This marks the second original clinical research paper published in JAMA by Zhongshan Ophthalmic Center, Sun Yat-sen University, and the second clinical study in the field of ophthalmology from China published in this journal.
This study addresses the critical question of the optimal number of cycles for postoperative adjuvant chemotherapy in children with high-risk retinoblastoma. The results demonstrate that the 3-cycle CEV regimen achieves equivalent efficacy to the traditional regimen. This finding holds promise for benefiting more children with high-risk retinoblastoma and potentially being incorporated into clinical guidelines.
Research Content and Results
This prospective, noninferiority randomized clinical trial enrolled 187 children with unilateral retinoblastoma who had undergone enucleation and exhibited high-risk pathologic features. The children were randomly assigned to receive either 3 cycles or 6 cycles of CEV chemotherapy. The primary outcome measure was disease-free survival (DFS). Secondary outcomes included overall survival, safety, economic burden, and quality of life.
The results showed that regarding the primary outcome (5-year DFS), the rates were 90.4% in the 3-cycle group and 89.2% in the 6-cycle group, with no significant difference. Furthermore, there was no statistical difference in 5-year overall survival between the two groups (91.5% vs 89.3%).
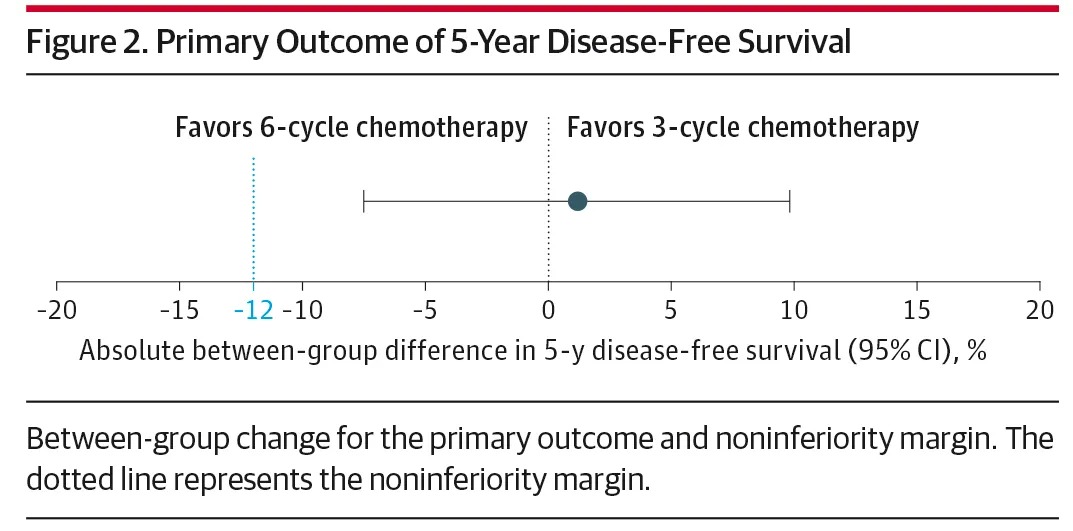
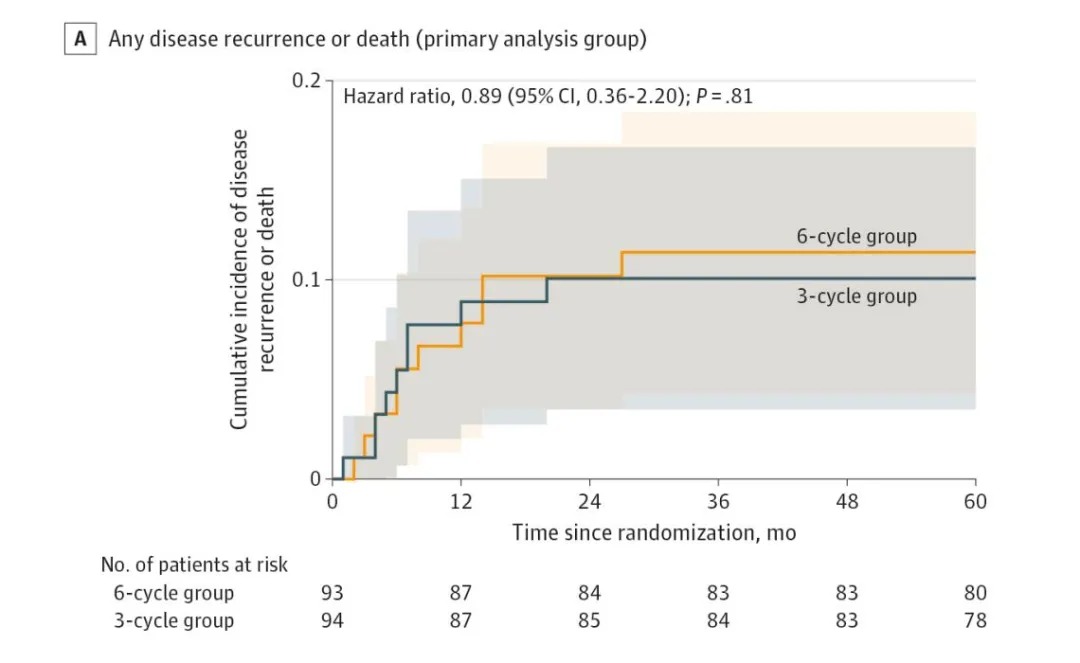
No significant difference in disease-free survival rate between the 3-cycle group and the traditional 6-cycle group.
In terms of treatment safety, 79 children (80.6%) in the 3-cycle group experienced a total of 282 adverse events. In contrast, 89 children (95.7%) in the 6-cycle group experienced a total of 681 adverse events. Additionally, the direct and indirect costs were significantly lower in the 3-cycle group compared to the 6-cycle group.
Research Significance
This study confirms that a 3-cycle CEV chemotherapy regimen is as effective as a 6-cycle regimen in preventing metastasis and reducing mortality for children with high-risk retinoblastoma, while also causing fewer side effects and lowering costs.
This finding could establish a new standard of care for retinoblastoma with high-risk pathologic features, potentially benefiting more children and being adopted into international guidelines.

JAMA gave high praise to this study, recognizing its significant implications for the treatment of rare tumors. Professor Carol Lally Shields, a leading international ocular oncologist, commented that the 3-cycle CEV regimen is a "landmark" development, as it "saves lives, reduces financial burden, and improves completion rates."
Author Information
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science served as the first author affiliation and the corresponding author affiliation for this study. Professor Yang Huasheng from ZOC and Professor Qian Jiang from the Eye, Ear, Nose, and Throat Hospital of Fudan University are the co-corresponding authors. Associate Chief Physician Ye Huijing from ZOC and Associate Chief Physician Xue Kang from the Eye, Ear, Nose, and Throat Hospital of Fudan University are the co-first authors.
Original Article Link:
https://jamanetwork.com/journals/jama/article-abstract/2825162